Advanced Therapies 2024: Driving Innovation in Cell and Gene Therapies

Posted by:
Alexis Tzannis
Published on:
Mar 1, 2024
The innovative field of cell and gene therapies (CGT) is evolving at a phenomenal rate and has enormous potential to transform medicine and patient treatment. By targeting the root cause of diseases at the genetic and cellular level, personalized therapies can be developed that help to address unmet medical needs.
We’re looking forward to attending Advanced Therapies 2024 in London, March 19–20
Advanced Therapies 2024 brings together forward-thinking global leaders in all aspects of CGT development. The congress covers the entire CGT value chain and provides a unique chance to explore the latest cutting-edge technologies. In addition to topics such as the pros and cons of autologous versus allogeneic therapies, the role of CAR cell therapy in the fight against solid tumors, and the complexities of cell therapy manufacture, the congress also highlights the role of automation and closed systems in all processes and manufacturing.
CGT workflows are highly complex
The cell material provided by patients is extremely valuable, and for many, a second cell draw poses a high risk to their health. Therefore, rigorous quality checks in the Cell and Gene Therapy (CGT) process are crucial to ensure the production of safe and reliable treatments.
Unlike traditional methods, where a single quality check sufficed for large batches, personalized therapies require individualized quality control for each patient's sample. The unique nature of these advanced therapies introduces new quality control standards that must be automated and significantly scaled up to ensure reproducibility and reliability of treatments for individual patients.
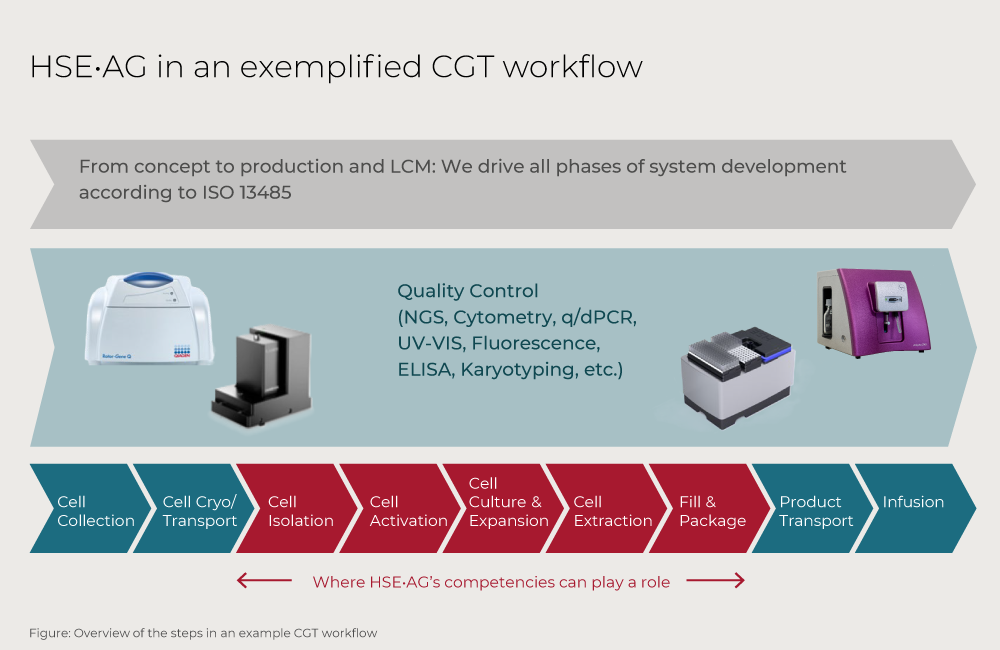
We can integrate automation into your CGT workflow
We are pioneers in automation of life science and diagnostics workflows offering end-to-end solutions for CGT platforms, including cell isolation and selection, quality control and beyond. Our applications expertise covers a wide range of technologies such as capillary electrophoresis, real-time PCR, NGS, and flow cytometry.
We develop according to ISO 14385 covering product ideation, development, industrialization, lifecycle management, and assembly and testing with a focus on mechanics, optics, electronics and software and algorithms.
For businesses and innovators looking to unlock the potential of CGT, the journey from concept to commercialization can be filled with challenges. Having a seasoned expert by your side – with our key technology and application knowledge – can greatly simplify this process.
Meet our expert, Alexis Tzannis, at Advanced Therapies 2024
If you’re curious and want to explore how automation can advance your CGT development, Alexis Tzannis and Martin Galic, two of our leading experts, will be at Advanced Therapies 2024 from March 19 to 20 in London, UK.
This is a great opportunity to discuss, collaborate, and gain insights that could pave the way for commercialization of your CGT product.
Contact Alexis Tzannis to meet him at the event.
