CAR T-Cell Therapy: The Next Frontier

Posted by:
Fabian Eckermann
Published on:
Jul 31, 2025
CAR T-cell therapy is an innovative technology that has transformed the treatment landscape for blood cancers. And this is only the beginning. Reprogramming a patient’s own immune cells is paving the way for a new era in personalized medicine.
CAR T-cells: The next frontier
What started as a groundbreaking treatment for certain types of leukemia, lymphoma, and multiple myeloma is now laying the foundation for a new era of personalized medicine. Researchers are continuously pushing the boundaries and the advancements mean CAR T-cell therapy is poised to move beyond blood cancers.
Unlocking the potential of CAR T-cells for solid tumors
One of the most urgent challenges is to enhance the effectiveness of CAR T-cell therapy. A major priority is patients with solid tumors as these make up the vast majority of cancer patients. Unlike blood cancers, solid tumors introduce a complex set of challenges.
- The hostile tumor microenvironment (TME) inhibits immune activity and presents a barrier to T-cell infiltration.
- Antigen expression in solid tumors tends to be highly heterogeneous, reducing the likelihood that CAR T-cells will recognize and attack cancer cells.
- Persistent antigen exposure within the tumor can lead to chronic stimulation of CAR T-cells, causing them to become exhausted and ineffective.
Innovative advancements using the powerful combination of CAR T-cell therapy and immune checkpoint inhibitors may help to increase long-term persistence, overcome exhaustion, and survive and thrive in the immunosuppressive tumor microenvironment.
New targets for CAR T-cells
CAR T-cell therapies are expanding into new disease areas. Clinical trials demonstrate that these therapies show promise in:
- Autoimmune diseases such as systemic lupus erythematous, myasthenia gravis, and type 1 diabetes.
- Infectious diseases such as HIV.
- Cardiovascular diseases such as cardiac fibrosis.
Engineering smarter CAR T-cells
New techniques, such as base editing, enable genetic modification of the DNA of CAR T-cells to enhance their efficacy, specificity, and safety.
Base editing introduces single nucleotide changes into a DNA sequence. Unlike the conventional CRISPR/Cas9 method, base editing does not create a double-strand break in the DNA. Instead, it uses a deaminase to introduce a point mutation into the DNA sequence.
Ready-to-use CAR T-cell therapy
Significant progress is being made in the development of “off-the-shelf” CAR T-cell therapies and clinical trials are testing CAR T-cell therapies using T-cells collected from healthy donors instead of individual patients. In contrast to the weeks-long manufacturing process required for individual patients, the use of donated, or allogeneic, T-cells allows CAR T-cell therapies to be prepared in advance and made immediately available to any patient.
These universal T cells, engineered to evade host immune detection and prevent graft-versus-host disease using gene editing technologies, promise scalable, cost-effective therapies with reduced manufacturing time.
Boosting CAR T-cell therapy with checkpoint inhibitors
CAR T-cell therapy and immune checkpoint inhibitors both harness the power of the immune system to fight cancer, but they work in different ways. CAR T-cells target and eliminate cancer cells and immune checkpoint inhibitors block proteins that cancer cells use to evade the immune system. This essentially releases the immune system’s brakes and significantly increases the treatment efficacy.
Why automation matters
The production of CAR T-cell therapies relies heavily on manual, labor-intensive procedures and requires highly skilled lab personnel. Without automation, it will not be possible to make CAR T-cell therapy available to as many cancer patients as possible. The production rates of existing methods are also far too low for widespread use.
Intuitive automated systems make a lot of manual work superfluous, reduce the training requirements for operating personnel, and minimize human error.
CAR T-cell therapies require individualized quality control for each patient’s sample. This introduces new quality-control standards that must be automated and significantly scaled up to ensure reproducibility and reliability of treatments for individual patients. Automating these verification assays can save as much time as automating the production.
Redefining what is possible in life sciences and diagnostics
Automating personalized therapies, such as CAR T-cell therapy, is particularly challenging. A large number of complex devices are needed throughout the multi-step process.
Furthermore, the cell material provided by patients is also extremely valuable and the reproducibility and reliability of treatments must be ensured.
We have a deep understanding of the automation needed for the various steps in the process, from T-cell selection and genetic modification to cell expansion and infusion. Our unique approach overcomes the challenges as we always think about device technology and biological processes together.
This mutual understanding between engineers and scientists is crucial for the development of a successful diagnostic device.
Don’t miss the other articles of our comprehensive blog series that focuses on the breakthroughs, challenges, and new horizons of CAR T-cell therapy.
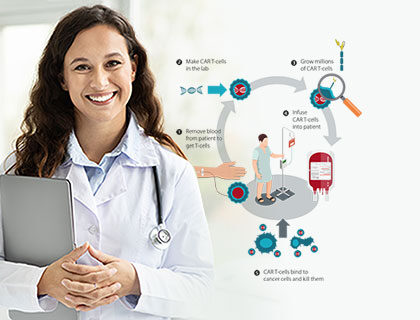
What exactly is CAR T-Cell Therapy?
CAR T-cell therapy is a form of immunotherapy against cancer in which the patient's own T-cells are genetically modified in the laboratory to recognise and attack cancer cells. The therapy aims to modify the immune system.

Revolutionizing Blood Cancer Treatment in CAR T-Cell Therapy
CAR T-cell therapy is a new treatment primarily used for certain types of blood cancers. This innovative approach is making significant strides in the medical field, offering hope to patients with challenging diagnoses.

Inside CAR T-Cell Therapy: Progress and Pitfalls
CAR T-cell therapy has revolutionized treatment of hematological malignancies, driving the enormous growth in CAR T-cell research. But while results are highly encouraging, the treatment involves multiple complex steps.

How Viral Vectors Drive CAR T-Cell Therapy
Viral vectors are essential for CAR T-cell therapy:
Viral vectors are at the heart of this cutting-edge technology and deliver the genetic information needed to turn T-cells into effective cancer killers.
