Microfluidics in Focus: The Power of Structured Functionalization

Posted by:
Alexis Tzannis
Published on:
Sep 2, 2025
The field of microfluidics has revolutionized the handling of liquids on the microscale enabling the development of cost-effective portable devices. Discover in the final part of our four-part series how to select and optimize structured surface functionalization methods. This article provides everything you need to know about the range of methods available, how to create sub-micron spots for analyte capture, challenges and solutions, and more.
Overview
Structured functionalization uses advanced techniques to precisely engineer the surface of the microfluidic device with microscale or even sub-micron capture sites. This allows close control of the surface chemistry and significantly improves efficiency, specificity, speed and throughput in genomics, proteomics, and clinical testing. This level of structured functionalization can be achieved using a variety of techniques, but it is important to take into account the challenges, considerations, and solutions.
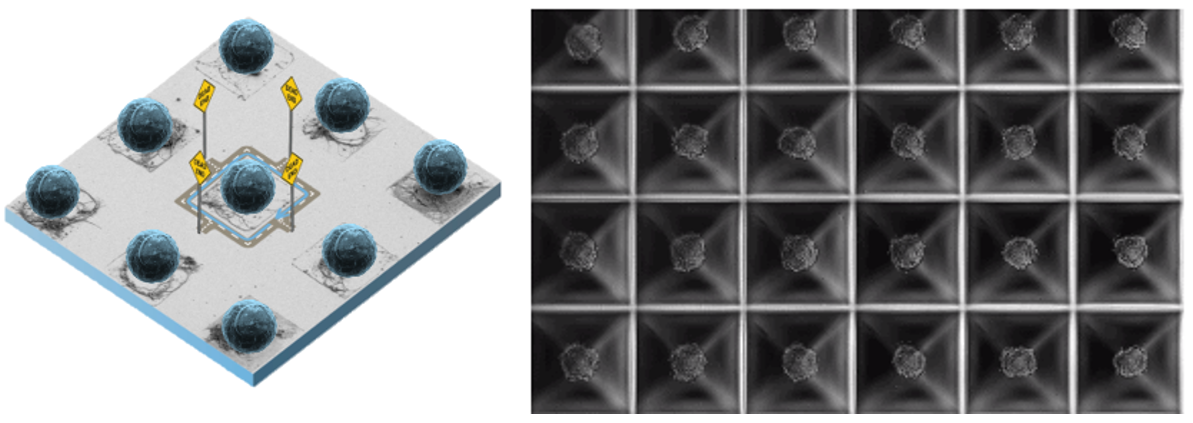
An essential requirement in advanced biosensing, diagnostics, and microfluidic applications is the ability to capture a variety of analytes by creating structured surface functional sites of (sub-)micron size, each bearing different capture chemistries. Achieving such precision allows for multiplexed detection of analytes with high sensitivity and specificity. Various methods, often combining multiple techniques, have been developed to accomplish this level of detail in surface functionalization.
Surface functionalization methods
Silanization
Silanization modifies substrate surfaces (e.g., glass, silicon, quartz) with organosilane reagents. These reagents, such as APTES (3-Aminopropyl)triethoxysilane, introduce functional groups (e.g., –NH₂, –SH, epoxy) by forming Si–O–Si bonds with the substrate. This forms a stable, reactive surface for subsequent bioconjugation, for instance for antibody immobilization (Wang et al. 2021).
Silanes for biomolecule immobilization:
1. Aminosilanes (e.g., APTES):
-
-
Provide primary amine groups for covalent coupling via EDC/NHS chemistry.
-
Useful for DNA and protein immobilization.
-
2. Epoxysilanes:
-
-
Enable nucleophilic reactions with biomolecules containing amines, thiols, or hydroxyl groups.
-
3. Thiolsilanes and maleimidosilanes:
-
-
Facilitate binding of thiol-containing molecules, such as cysteine residues or thiolated DNA.
-
PEGylation
PEGylation involves the application of polyethylene glycol (PEG) to surfaces to:
- Reduce non-specific binding.
- Enhance biocompatibility.
- Create a hydrophilic, anti-fouling surface for sensitive biomolecule applications, e.g., priming (BOC Sciences).
SuSoS of Switzerland has created a compelling list of surface-related topics for “Bonding anything to anything” that is definitely worth taking a look at.
SuSoS, AziGrip4™, and PAcrAm™ coatings provide highly durable non-fouling surfaces that prevent unspecific protein, cell, and biofilm attachment. These coatings form a stable hydrostatic barrier without using active agents, reducing background noise in diagnostics, improving organoid formation, and preventing clogging or contamination in microfluidics and bioreactors.
The polymeric layers withstand extreme pH, mechanical stress, ethanol, and even γ-sterilization, making them ideal for applications such as organ-on-chip platforms, 3D cell culture, and point-of-care diagnostics.
Formation of spheroids in special wells coated with AziGrip4™ EX.
Copyrights with the copyright holder (from SuSoS Functional Coatings Overview).
PEGylation as an alternative to silanization
- Direct PEGylation: Application of PEG coatings for non-specific binding reduction (BOC Sciences).
- Patterned PEGylation: Use of biotin-PEG or azide-PEG with lithographic techniques for localized functionalization.
Click chemistry
Click chemistry offers a highly efficient and specific method for attaching biomolecules to surfaces. It typically employs copper-catalyzed azide-alkyne cycloaddition (CuAAC), creating stable triazole linkages. This technique is particularly effective for modifying silanized or PEGylated surfaces, enabling precise and stable functionalization (Barua et al. 2021, SuSoS, Switzerland).
Click chemistry for functionalization
- Silanized Surfaces: Functionalize silanized substrates with azide or alkyne groups for subsequent click chemistry reactions (Wang et al. 2021).
- PEGylated Surfaces: Conjugate alkyne-PEG to azide-modified surfaces, achieving both specificity and anti-fouling properties (BOC Sciences).
...
Leave your data to access the full article.
The full article covers:
-
Core surface functionalization methods such as silanization, PEGylation, and click chemistry, including recommendations
-
Advanced patterning techniques including lithography, CVD, and aerosol jet printing
-
How structured surfaces enable multiplex analyte detection with high sensitivity and throughput
-
Key challenges like material compatibility, non-specific binding, and scalability — and practical solutions to address them
-
Case studies showing how these technologies are shaping diagnostics and organ-on-chip platforms
It’s a comprehensive guide full of insights and real-world examples that point to the future of microfluidics.
Leave your data here:
We support your success
As a global systems engineering provider with a proven track record in life science and diagnostics, we recognize your need for innovative solutions. Our deep microfluidics expertise, combined with a full understanding of your workflows, enables us to transform your vision into market-ready solutions that exceed customer expectations.
Contact Alexis Tzannis and discover how we can help you innovate faster and smarter.

Microfluidics Expert Alexis Tzannis
Alexis holds a PhD in physical chemistry from ETH Zürich, is fluent in four languages, and has a proven record of forging academia–industry partnerships to accelerate innovation. A founding member of the Microfluidics Association (MFA), he is a micro- and nanotechnology business leader with 15 years of experience taking microfluidic products from concept to industrialization.
Alexis has delivered double-digit sales growth at HSE•AG and previously at IMT Microtechnologies by leading cross-functional teams and cultivating global key accounts. His technical expertise spans the design and manufacture of glass, silicon, and hybrid-material microfluidic platforms that enable cutting-edge analytical applications, e.g., next-generation sequencing (NGS) and others.
